Acids React With Metals To Form
Acids React With Metals To Form. The reaction is called a neutralization reaction. What reacts with acids to form salts?

 PPT Metal Reactions and Reactivity PowerPoint from www.slideserve.com
PPT Metal Reactions and Reactivity PowerPoint from www.slideserve.comMetals react with acid to give hydrogen gas and form metal salts. As a result of the process, the elemental metals transform into metal cations due to oxidation. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a squeaky pop sound.

In instances where the nitric acid is dilute, nitrogen monoxide is. Most of the metals react with acids to form salt and hydrogen gas.
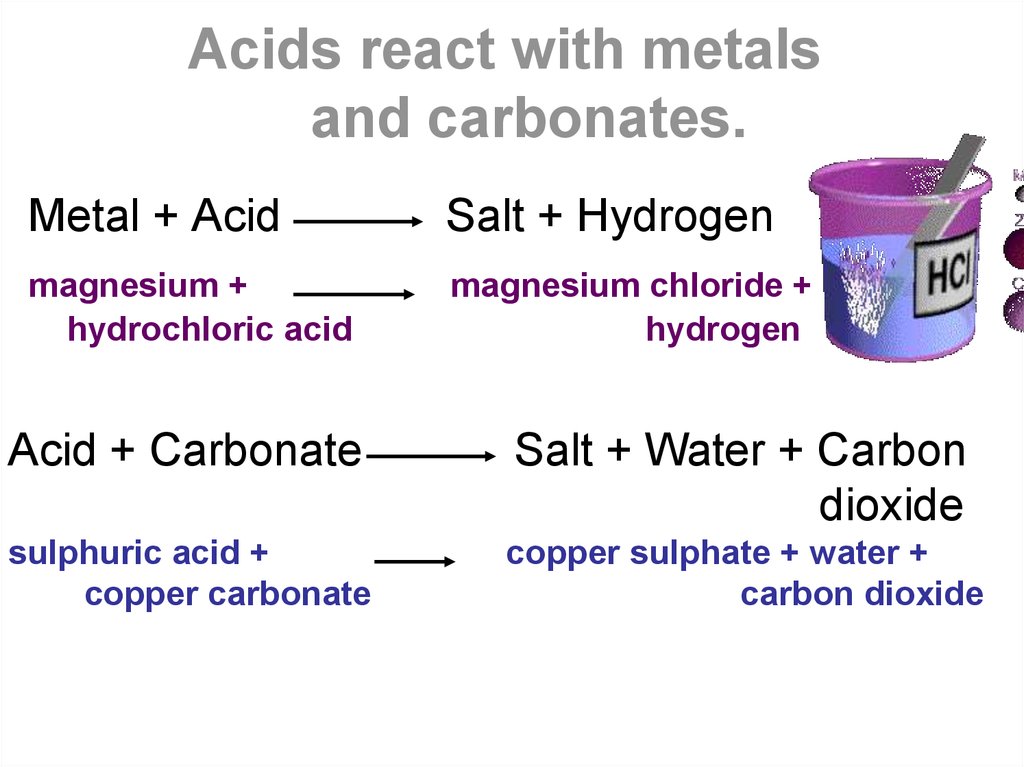
Metal + acid → salt + hydrogen the abbreviation m.a.s.h. During neutralization reaction acids react with.to form.and water.

This leads to a pop sound, indicating the evolution of hydrogen gas. Acids react with most metals to form a salt and hydrogen gas.

Metal oxides and hydroxides as bases. We have seen that, some metals react immediately and vigorously with reactants, while others react slowly , or when the reaction mixture is heated/catalysed.
The evolution of hydrogen gas can be confirmed by bringing a lit candle near the mouth of the test tube. Acids react with certain metals, such as magnesium, zinc, and iron, to produce hydrogen gas.

What reacts with acids to form salts? 2koh + h 2 so 4 → k 2 so 4 + 2h 2.

Metals react with acids to produce salt and hydrogen | acid & bases | chemistry. Metals react with dilute acids to form metallic salts and hydrogen gas.
As a result of the process, the elemental metals transform into metal cations due to oxidation. The general word equation for the reaction between an acid and a metal is as follows:
Metal + base → hydrogen + salt. For each type of reaction, the balanced equation, which means the number of atoms on the reactant side (left side of the arrow) equals the.

Can be used to remember this general reaction. The reaction is called a neutralization reaction.
Alkali + metal → salt + hydrogen. If a matchstick is brought near the mouth of the tube containing the product of the reaction then we hear a pop sound.
Acids react with certain metals, such as magnesium, zinc, and iron, to produce hydrogen gas. All acids react with alkalis (metal hydroxides) to form salt and water.
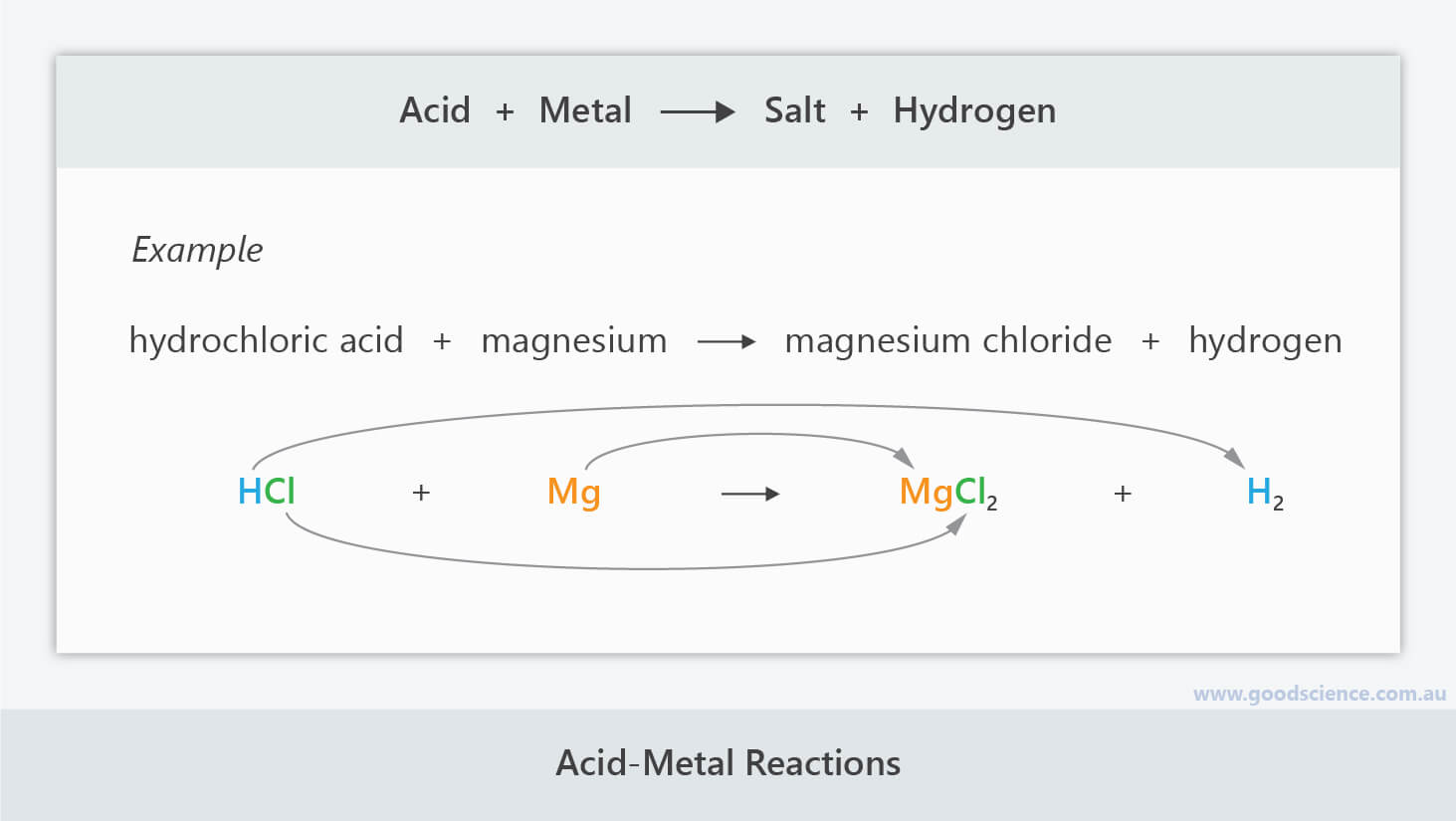
When zinc (zn) reacts with dilute sulphuric acid (h 2 so 4 ), it produces a salt called zinc sulphate (znso 4 ) and hydrogen gas. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a squeaky pop sound.

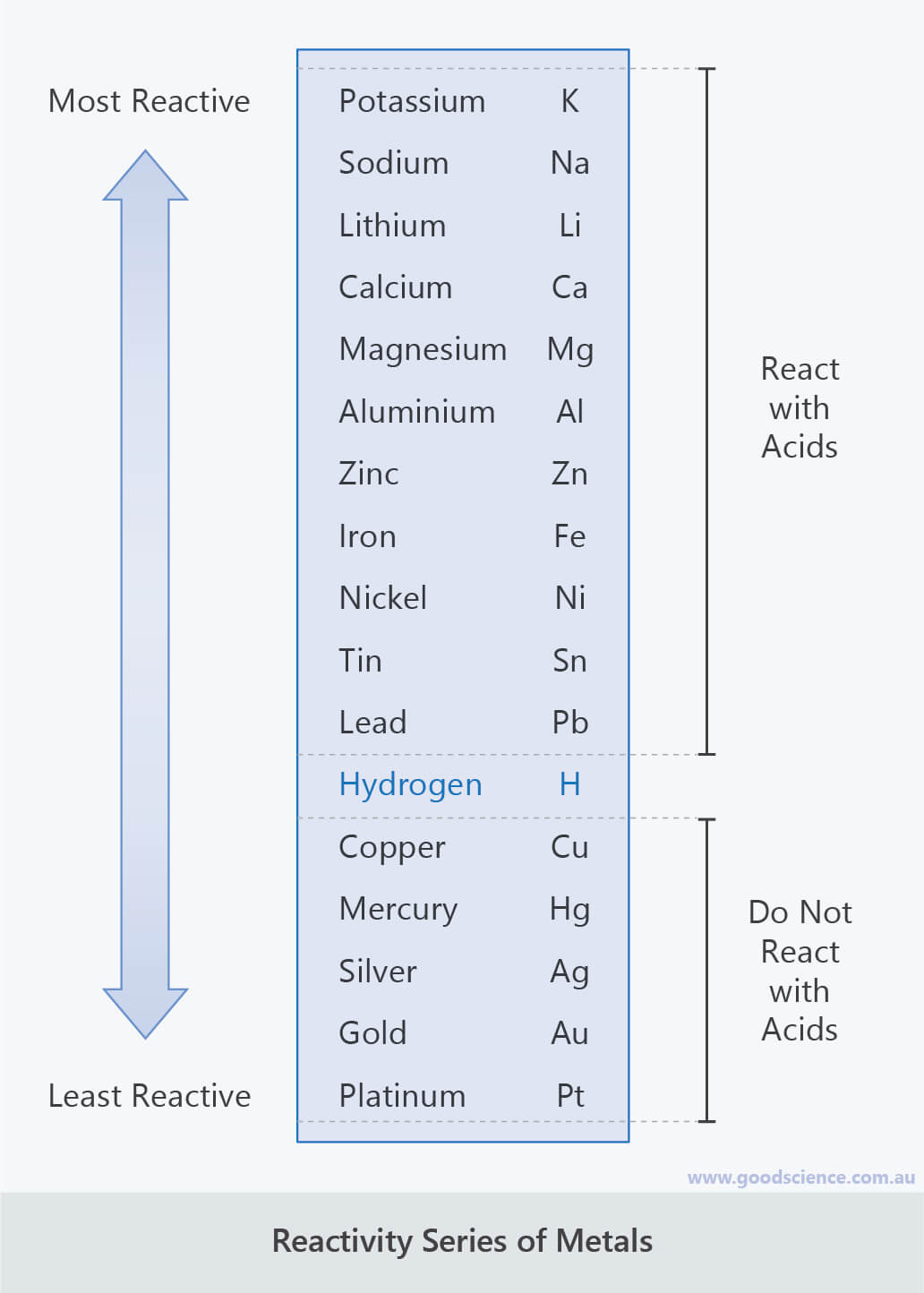
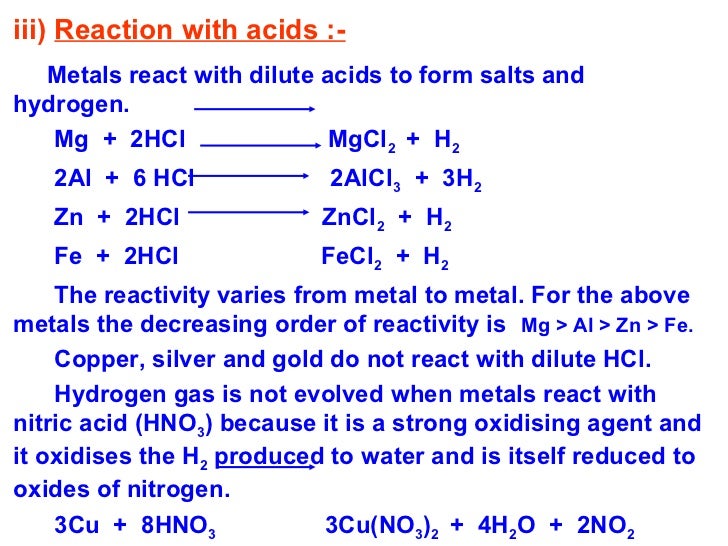
Only the less reactive metals like copper,silver and gold do not react with dilute acids. 2al(s) + 6hcl(aq) 2alcl 3 (aq) + 3h 2 (g).

Acids react with most metals to form a salt and hydrogen gas. If a matchstick is brought near the mouth of the tube containing the product of the reaction then we hear a pop sound.
A base reacts with a metal to form salt. For each type of reaction, the balanced equation, which means the number of atoms on the reactant side (left side of the arrow) equals the.
Reaction of metals with acids. What reacts with acids to form salts?
Metal + base → hydrogen + salt. During neutralization reaction acids react with.to form.and water.

An acid will react with a metal to form a salt and hydrogen gas. Metal oxides and hydroxides as bases.

It is this hydrogen gas that burns with a pop sound. For example, zinc metal reacts with hydrochloric acid producing zinc chloride and hydrogen gas.
Besides Corroding Metallic Structures, Acid Rain Also Gnaws Away Stone Monuments Made Up Of Limestone, Which Is Essentially Calcium Carbonate.Reactivity is the tendency of chemical substances to form products by itself. The general word equation for the reaction between an acid and a metal. Metal + base → hydrogen + salt.
Mg(S) + H 2 So 4 (Aq) Mgso 4 (Aq) + H 2 (G) The Reaction With Dilute Hydrochloric Acid Looks Exactly The Same, But This Time Magnesium Chloride Is Produced.Reaction of metals with acids. This leads to a pop sound, indicating the evolution of hydrogen gas. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a squeaky pop sound.
All Acids React With Alkalis (Metal Hydroxides) To Form Salt And Water.Identify the nature of metal oxide and non metal oxide. When a base reacts with a metal, hydrogen gas is produced. The general word equation for the reaction between an acid and a metal is as follows:
The Colour Of The Indicator Changes When All The Acid Has Reacted With The Soluble Solution Of The AlkaliAcidic, basic, and amphoteric oxides. 2al(s) + 6hcl(aq) 2alcl 3 (aq) + 3h 2 (g). The reaction of an acid with metal oxides/hydroxides (bases) to salt and water only is called neutralization reaction.
Reactions Taking Place Between Acids And Bases:Bases are known to turn blue on red litmus paper. Acids react with certain metals, such as magnesium, zinc, and iron, to produce hydrogen gas. This reaction can be easily represented by the following word equation:
Belum ada Komentar untuk "Acids React With Metals To Form"
Posting Komentar