Strong Acids And Weak Bases
Strong Acids And Weak Bases. Because mg(oh) 2 is listed in table 12.2 "strong acids and bases", it is a strong base. This does not however mean that weak acids fail to react fully with bases.

 List of Common Strong and Weak Acids from sciencenotes.org
List of Common Strong and Weak Acids from sciencenotes.orgStrong acid is an acid that ionize completely while weak acid partially ionize. 6 strong acids and 6 strong bases; Compounds containing ionizable hydrogen or hydroxide ions can be an acid or base.
/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)
Strong acids/bases dissociate completely whereas weak acids/bases dissociate partially. Many bases, such as lime water, can also be found.
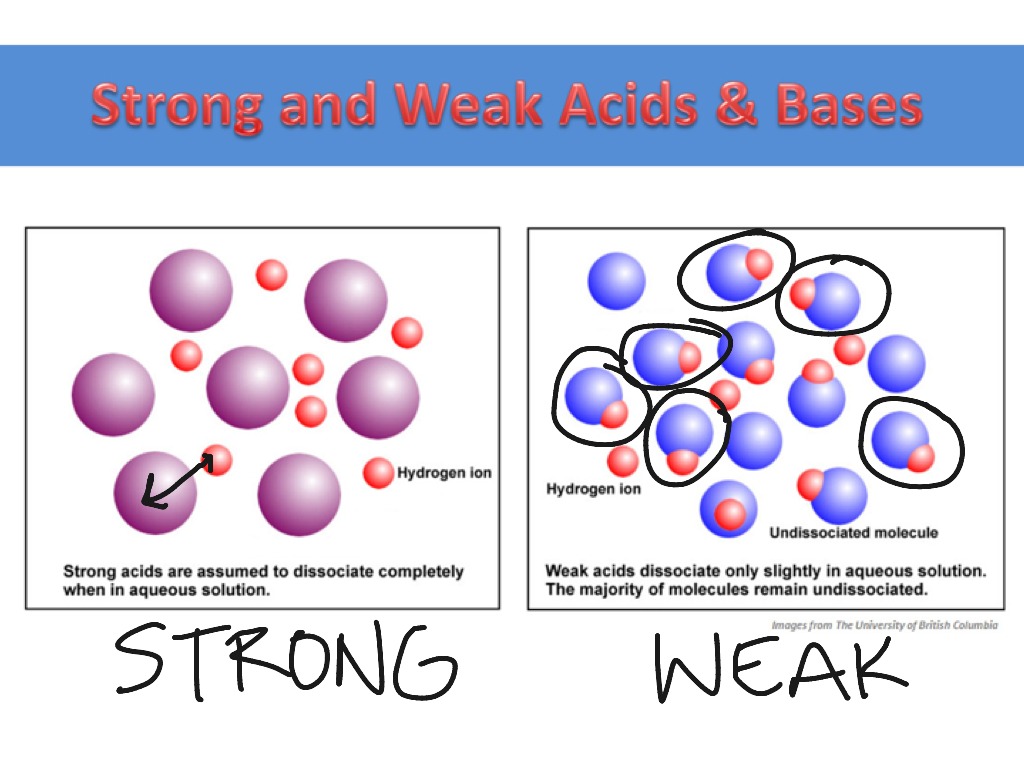
Hclo 3, hbro 3, hio 3, h 2 seo 4 assume all other acids are weak unless told otherwise. Strong and weak acids and bases 1.
Weak acids and weak bases will neutralize each other if their if their ionization constants are equal. Get instant access to all materials become a member.
Lesson 5, unit 5 chemistry a: Strong and weak acids and bases 1.
22 votes) whenever any acid is mixed with base, there is a change in ph of the solution. Mg(oh) 2 c 5 h 5 n;

In this video, i will teach you step by step the strength of acids and bases to the point where you will be able to go out answer exam questions on strong ac. Many bases, such as lime water, can also be found.
I) strong acids and ii) weak acids strong acids. We've learned that the conjugate acid of a base is the molecule that is formed when.
The bases which undergo partial ionisation in aqueous solution are called weak bases. Remember that neutralization is the reaction:

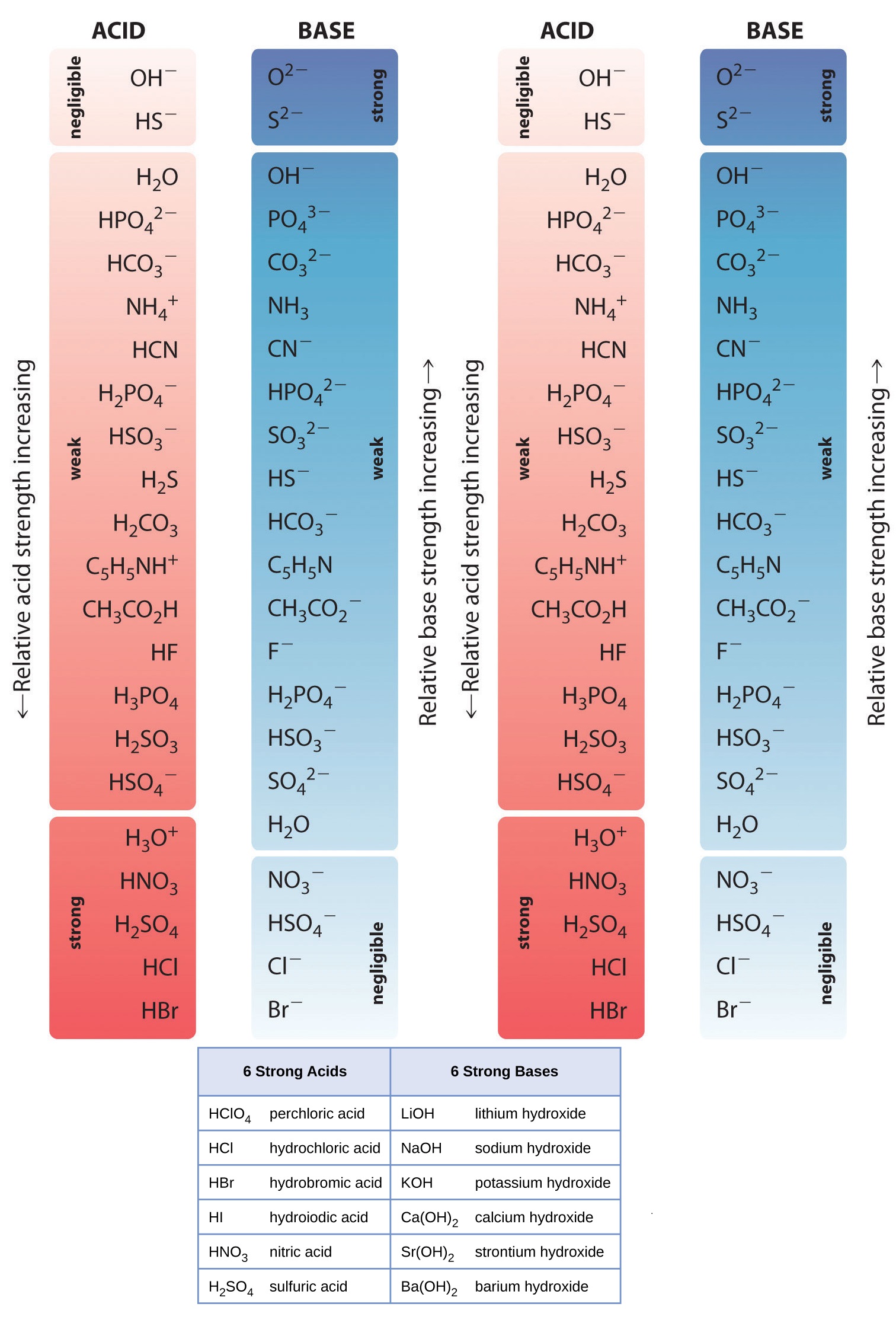
Compounds containing ionizable hydrogen or hydroxide ions can be an acid or base. 6 strong acids and 6 strong bases;
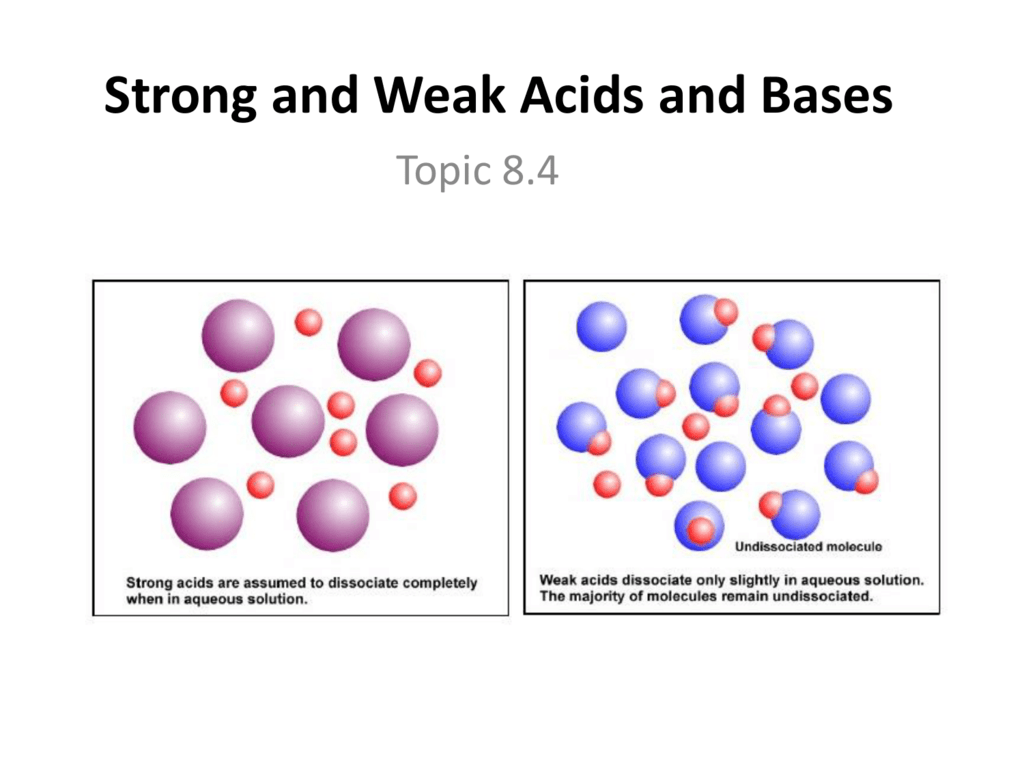
Strong and weak acids and bases 1. Because hcl is listed in table 12.2 "strong acids and bases", it is a strong acid.;

Strong acid add all their h+ to will weak acid only add some h+ to solution. N h 4 o h, n h 3.etc
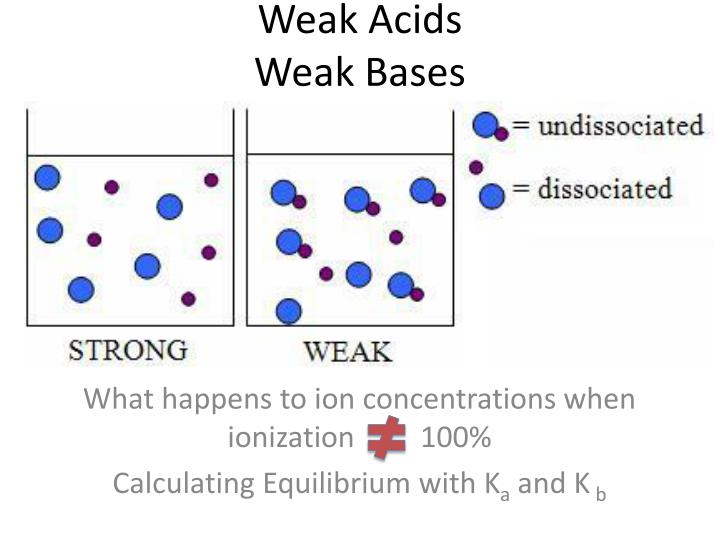
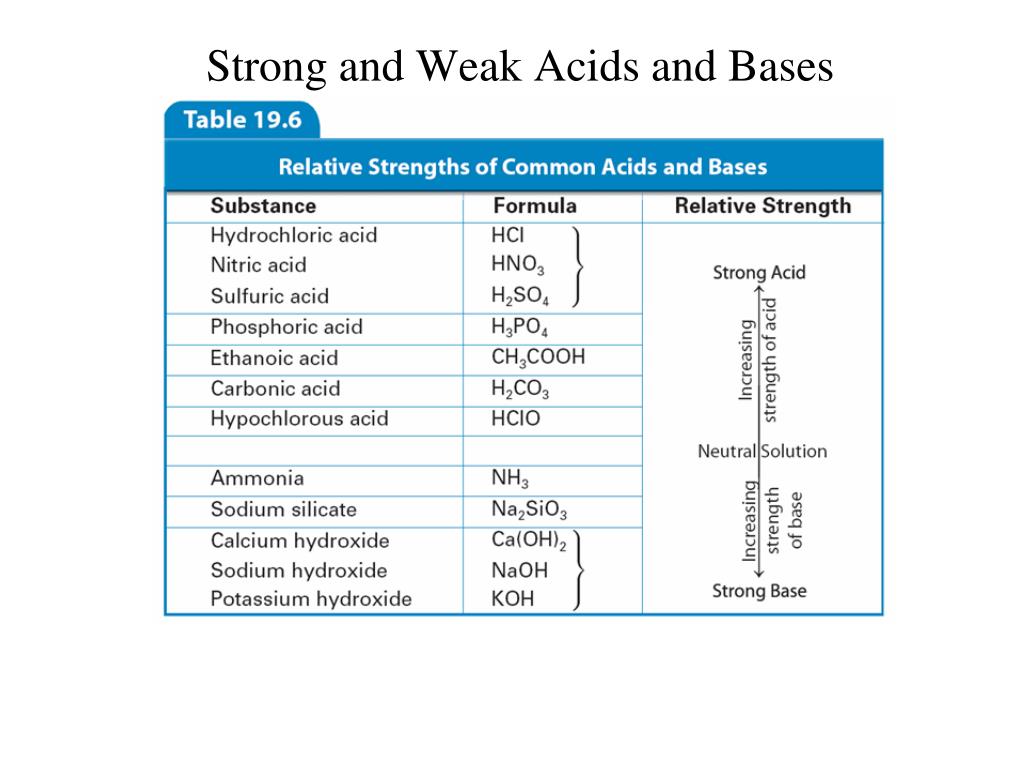
Acids have ph values from 1 to 7. Hf, hno 2, hclo 2, [h 2 so 3] = so 2 + h 2 o, hc 2 h 3 o 2 = hoac 2.
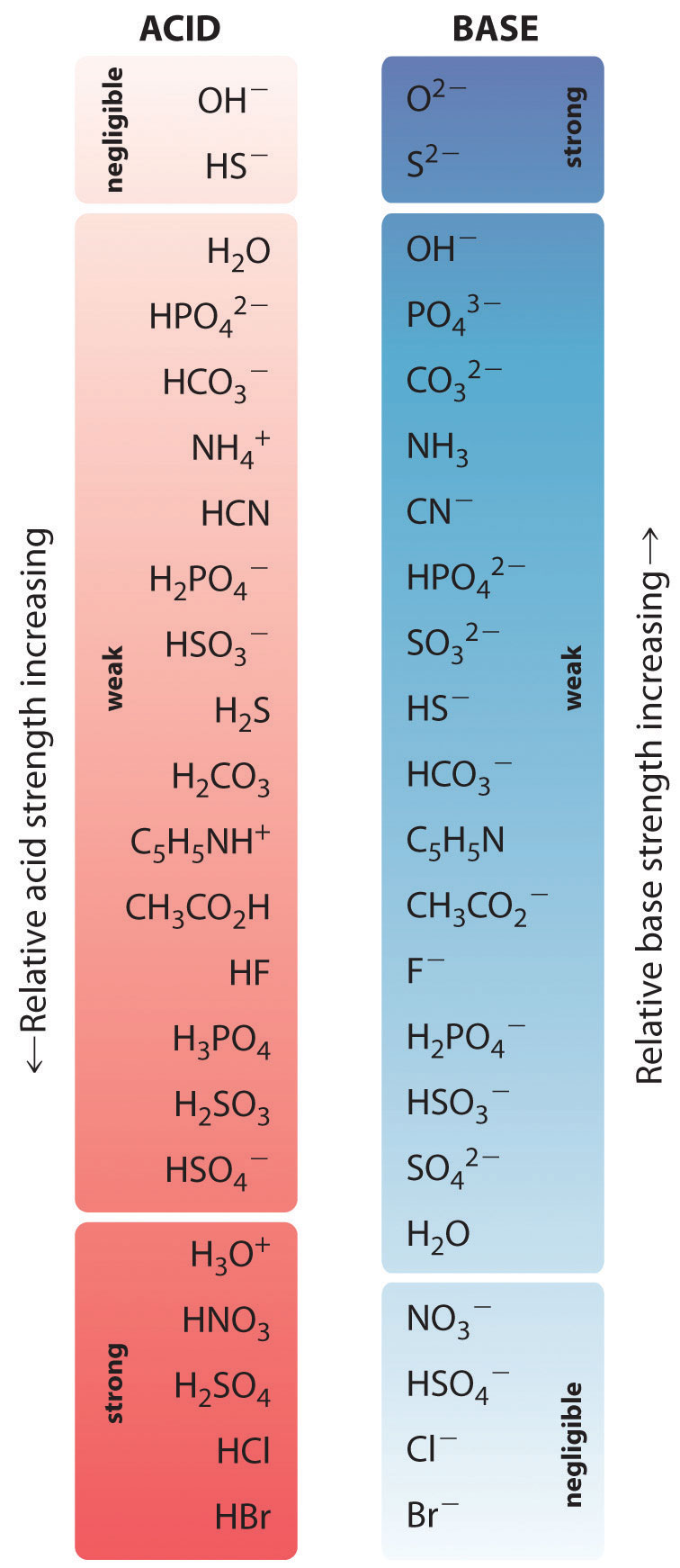
Hcl, hbr, hi, hno 3, hclo 4, h 2 so 4 the following are some less common acids that are also strong: Weak acids, weak bases, and salts;
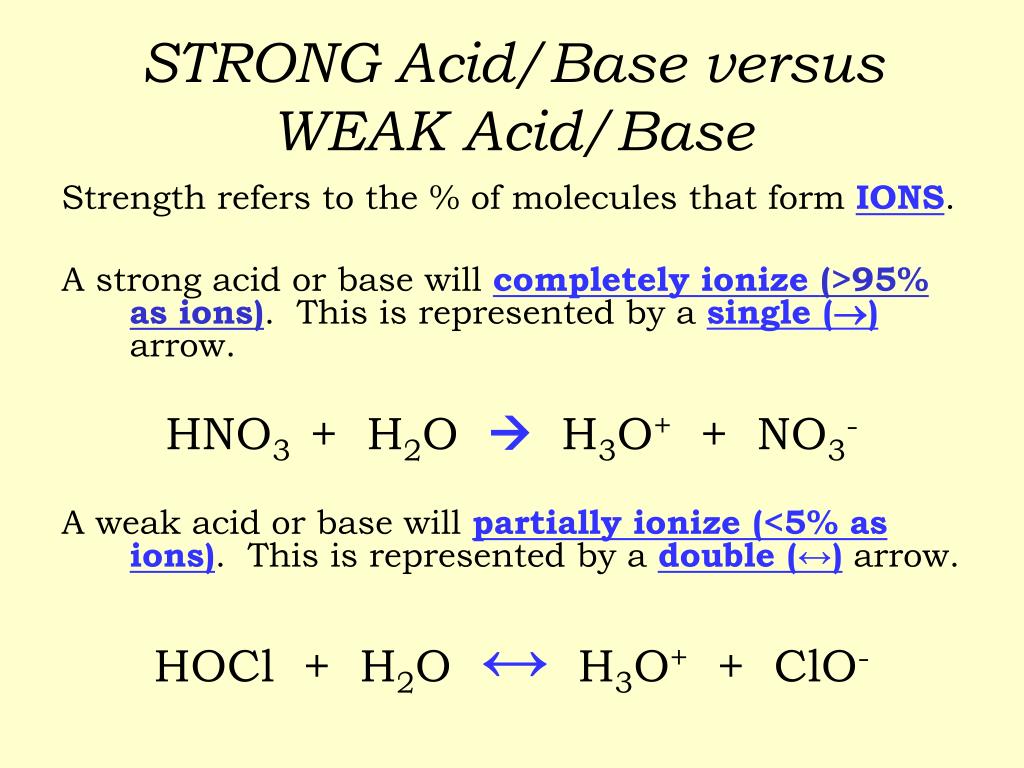
This does not however mean that weak acids fail to react fully with bases. Ammonia itself obviously doesn't contain hydroxide ions, but it reacts with water to produce ammonium ions and hydroxide ions.
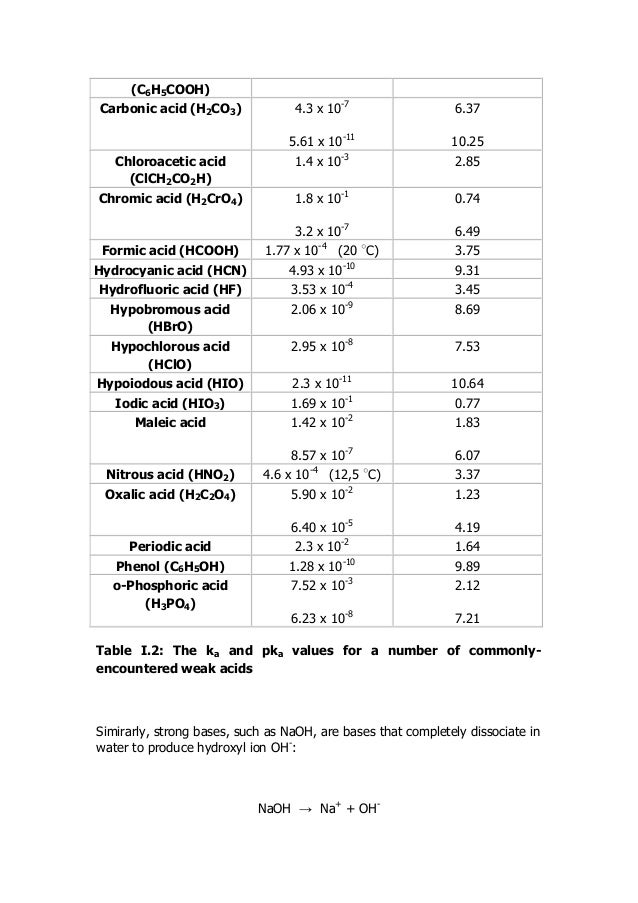
Get instant access to all materials become a member. Effect of ph on strength of acids and bases.
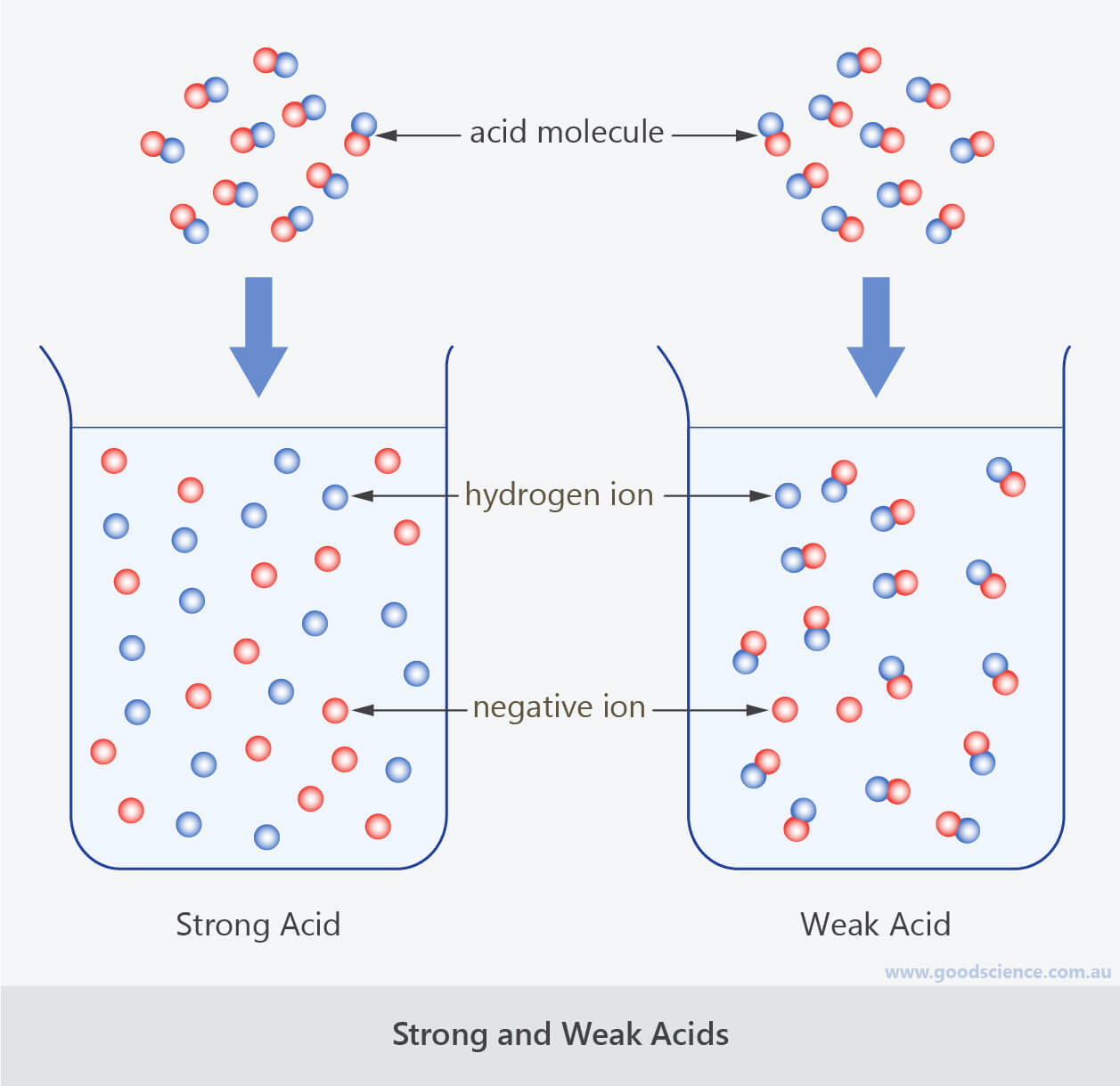
Dissolves and dissociates 100% to produce protons (h+) 1. The bases which undergo partial ionisation in aqueous solution are called weak bases.

Lesson 5, unit 5 chemistry a: 6 strong acids and 6 strong bases;
Strong acid add all their h+ to will weak acid only add some h+ to solution. Strong acids/bases dissociate completely whereas weak acids/bases dissociate partially.
The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid. Hclo 3, hbro 3, hio 3, h 2 seo 4 assume all other acids are weak unless told otherwise.

Weak acids, weak bases, and salts; All the other acids are weak.
Hcl, Hbr, Hi, Hno 3, Hclo 4, H 2 So 4 The Following Are Some Less Common Acids That Are Also Strong:Because mg(oh) 2 is listed in table 12.2 "strong acids and bases", it is a strong base. In aqueous solution, each of these essentially ionizes 100%. The only weak acid formed by the reaction between hydrogen and a halogen is hydrofluoric acid (hf).
This Does Not However Mean That Weak Acids Fail To React Fully With Bases.Strong acid add all their h+ to will weak acid only add some h+ to solution. Ammonia itself obviously doesn't contain hydroxide ions, but it reacts with water to produce ammonium ions and hydroxide ions. Strong and weak acids and bases (please see page 4 of this article for clickable links) acids are divided into two categories based on the ease with which they can donate protons to the solvent:
All The Other Acids Are Weak.One of the other differences between strong and weak acids and bases is in measurements like the enthalpy of neutralization. N h 4 o h, n h 3.etc This is thoroughly answered here.
Acids And Bases That Are Dissociated To A Limited Extent Giving A Lesser Amount Of Hydrogen Or Hydroxide Ions In Solutions Are Termed Weak Acids And Bases.Hclo 3, hbro 3, hio 3, h 2 seo 4 assume all other acids are weak unless told otherwise. Strong acid is an acid that ionize completely while weak acid partially ionize. Get instant access to all materials become a member.
The Main Difference Between Strong And Weak Base Is That Strong Bases Can Completely Dissociate To Give All Available Hydroxyl Ions To The System Whereas Weak Bases Are Partially Dissociated To Give Some Of The Hydroxyl Ions It Has.The bases which undergo partial ionisation in aqueous solution are called weak bases. In other words, they do. Salts of weak acids or bases can affect the acidity or basicity of their aqueous solutions.
Belum ada Komentar untuk "Strong Acids And Weak Bases"
Posting Komentar